Background:
Richter Transformation (RT) refers to the development of an aggressive lymphoma in the setting of chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). Small molecule inhibitors (SMI), such as the Bruton tyrosine kinase inhibitors (BTKi) and BCL2 inhibitors (BCL2i), have revolutionized the treatment of CLL/SLL. Prior studies evaluated outcomes of patients (pts) with RT in an era where chemoimmunotherapy (CIT) was typically used to treat CLL/SLL. Now that SMI are standard of care, fewer pts with CLL/SLL receive CIT, therefore we sought to determine which variables predict survival in pts who developed RT without prior CIT exposure for CLL/SLL.
Methods:
We conducted an international multicenter retrospective study of pts from 11 academic centers. Pts with RT occurring as large B-cell lymphoma who did not previously receive CIT (e.g., fludarabine, cyclophosphamide, bendamustine, chlorambucil) for their CLL/SLL were included. We collected pt, disease, and treatment (tx) characteristics. RT was categorized into 3 groups: “Concurrent RT,” defined as RT and CLL/SLL diagnosed simultaneously (within 3 months [mos]); “RT w/o prior CLL/SLL tx,” defined as RT and CLL/SLL diagnosed >3 mos apart with the CLL/SLL never treated; and “RT with prior non-CIT tx for CLL/SLL” defined as having received prior CLL/SLL tx. Overall survival (OS) was measured from RT diagnosis (Dx) and estimated using the Kaplan-Meier method. Cox regression model was used to identify prognostic factors associated with OS.
Results:
242 pts were identified. At CLL/SLL Dx, 66% of pts had unmutated IGHV, 37% had del17p/ TP53 disruption, and 20% had trisomy 12. At RT Dx, 56% of the RT with prior non-CIT tx for CLL/SLL group had a del17p/ TP53 disruption versus 27% for the concurrent RT group and 18% for the RT w/o prior CLL/SLL tx groups.
Median time from CLL/SLL Dx to RT Dx was 47 mos (range 3-394) for RT w/o prior CLL/SLL tx and 46 mos (range 4-218) for RT with prior non-CIT tx for CLL/SLL. Pts with prior non-CIT tx for CLL/SLL received a median of 1 (range 1-5) prior tx. 86% of pts previously received a BTKi or a BCL2i for tx of CLL/SLL (54% BTKi only, 5% BCL2i only, 27% both). The remaining 14% had been treated with an anti-CD20 monoclonal antibody (MoAb), steroids, lenalidomide, radiation, or alemtuzumab. 85% of pts developed RT while on active tx with SMI.
Median age at RT Dx was 69 years (range 37-92). At RT Dx, 38% and 21% of pts had del17p/ TP53 disruption, and trisomy 12 in CLL/SLL tissue, respectively. At RT Dx, there was no difference in highest SUV on PET (median 16, range 2.9-65.6), LDH (median 325, range 103-8162, ULN range 180-271), largest lymph node in diameter (median 4.9 cm, range 0-17.1), Ki-67 (median 80%, range 5%-100%), rate of GCB subtype (27%), presence of MYC translocation (20%), or CLL/SLL and RT clonal relationship (81%) for those pts tested amongst the 3 groups ( Table).
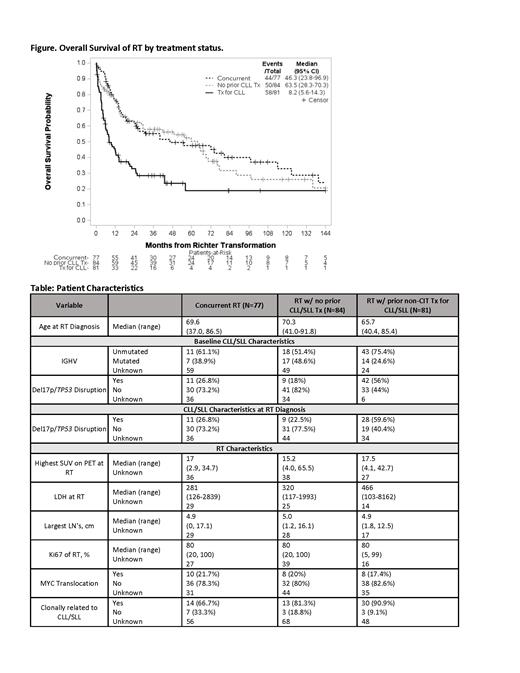
The most common 1 st line tx for RT was CIT with or w/o radiation and/or steroids (76%), followed by CIT plus SMI (13%), SMI single agent or combination (7%), and other (4%, which includes anti-CD20 MoAb). Median follow-up from RT Dx was 42.3 mos; the median OS for the entire cohort was 25.8 mos (95% CI: 16.8-49.1). Pts with RT after non-CIT tx for CLL/SLL had a significantly worse OS (median 8.2 mos [95% CI: 5.6-14.3]) than pts with concurrent RT (median 46.3 mos [95% CI: 23.8-96.9]) and RT w/o prior CLL/SLL tx (median 63.5 mos [95% CI: 28.3-70.3]) ( Figure).
From an exploratory univariable analysis, we found RT after non-CIT tx for CLL/SLL compared to concurrent RT (HR 2.32 [95% CI: 1.55-3.46]), RT Dx age (HR for 5-year increase 1.14 [95% CI: 1.05-1.23]), del17p/ TP53 disruption (HR 2.27 [95% CI: 1.45-3.56]), and LDH (HR for 2-fold increase 1.37 [95% CI: 1.20-1.58]) were prognostic for worse OS.
Conclusions:
This is the largest cohort of pts who developed RT having received only SMI with no CIT for their CLL reported, thus reflecting the current treatment landscape. Despite being CIT-naïve, pts in our series who received tx for their CLL/SLL and then developed RT had a short OS, underscoring the need to develop better therapies for these pts. In contrast, as in other published analyses (Wang Haematologica 2020), pts w/o prior tx for CLL/SLL had more favorable outcomes, with a median OS of approximately 5 years. Further analysis to determine PFS and OS by tx for RT for pts who have been treated with SMI for their CLL/SLL is ongoing.
Disclosures
Kittai:Abbive: Consultancy; AstraZeneca: Consultancy, Research Funding; BeiGene: Consultancy, Research Funding, Speakers Bureau; Eli Lilly: Consultancy; Janssen: Consultancy; KITE: Consultancy; BMS: Consultancy. Allan:TG Therapeutics, Inc: Consultancy, Research Funding; Janssen: Consultancy, Research Funding, Speakers Bureau; Lava Therapeutics: Consultancy; Pharmacyclics LLC: Consultancy, Speakers Bureau; Lilly: Consultancy; Adaptive Biotechnologies: Consultancy; BeiGene: Consultancy, Honoraria, Research Funding, Speakers Bureau; Epizyme: Consultancy; AbbVie: Consultancy, Honoraria, Speakers Bureau; ADC Therapeutics SA: Consultancy; Genentech, Inc.: Consultancy, Research Funding; AstraZeneca: Consultancy, Honoraria. Bhat:Aptitude Health: Honoraria; Abbvie: Consultancy; AstraZeneca: Consultancy, Research Funding. Bond:Novartis: Consultancy, Research Funding; Nurix Therapeutics: Consultancy, Research Funding; SeaGen: Consultancy; Incyte: Research Funding. Brander:Juno/Celgene/BMS: Other: Site PI clinical trial (grant paid to institution, Research Funding; Beigene: Other: Site PI clinical trial (grant paid to institution), Research Funding; MEI Pharma: Other: Site PI clinical trial (grant paid to institution), Research Funding; Ascentage: Other: Site PI clinical trial (grant paid to institution), Research Funding; Novartis: Other: Site PI clinical trial (grant paid to institution), Research Funding; Catapult: Other: Site PI clinical trial (grant paid to institution), Research Funding; DTRM: Other: Site PI clinical trial (grant paid to institution), Research Funding; Pharmacyclics: Consultancy, Other: Site PI clinical trial (grant paid to institution), Research Funding; ArQule/Merck: Other: Site PI clinical trial (grant paid to institution), Research Funding; AstraZeneca/Acerta: Other: Site PI clinical trial (grant paid to institution), Research Funding; NeWave: Other: Site PI clinical trial (grant paid to institution), Research Funding; AbbVie: Consultancy, Other: Site PI clinical trial (grant paid to institution), Research Funding; Genentech: Consultancy, Other: Site PI clinical trial (grant paid to institution), Research Funding; TG Therapeutics: Other: Site PI clinical trial (grant paid to institution), Research Funding; Pharmacyclics: Other: Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid for NCCN panel member CLL/SLL and HCL, informCLL registry steering committee; AbbVie: Other: Core registry steering committee ; CLL Society: Other: Alliance in Clinical Trials: Leukemia committee member & Trial Champion of S1925 . Byrd:Eilean Therapeutics: Consultancy, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees, Research Funding; Newave: Membership on an entity's Board of Directors or advisory committees, Research Funding; Kurome: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Vincerx: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; OSU Drug Devel. Inst.: Consultancy; Orbimed: Consultancy, Research Funding; Orange Grove Bio: Membership on an entity's Board of Directors or advisory committees; American Cancer: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Other: TRAVEL, ACCOMMODATIONS, EXPENSES. Chavez:Genmab: Honoraria; Epizyme: Speakers Bureau; Cellectar: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Beigene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astra Zeneca: Research Funding; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive: Research Funding; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Membership on an entity's Board of Directors or advisory committees; Lilly: Honoraria; Merck: Research Funding; Morphosys: Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees. Chong:MJH Healthcare Holdings, LLC: Honoraria; Novartis: Honoraria; Genentech: Research Funding; Abbvie: Research Funding; BMS: Honoraria; Beigene: Honoraria. Davids:Research to Practice: Consultancy; Mingsight Pharmaceuticals: Consultancy; Merck: Consultancy; Janssen: Consultancy; Genentech: Consultancy, Research Funding; Eli Lilly: Consultancy; Curio Science: Consultancy; BMS: Consultancy; BeiGene: Consultancy; Aptitude Health: Consultancy; Adaptive Biosciences: Consultancy; Ascentage Pharma: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Surface Oncology: Research Funding; Novartis: Research Funding; Secura Bio: Consultancy; TG Therapeutics: Consultancy, Research Funding; Takeda: Consultancy; MEI Pharma: Research Funding; ONO Pharmaceuticals: Consultancy; AbbVie: Consultancy, Research Funding. Danilov:GenMab: Consultancy, Research Funding; Astra Zeneca: Consultancy, Research Funding; Lilly Oncology: Consultancy, Research Funding; Nurix: Consultancy, Research Funding; Beigene: Consultancy, Research Funding; Merck: Consultancy; Abbvie: Consultancy, Research Funding; Bayer: Research Funding; Cyclacel: Research Funding; MEI: Consultancy, Research Funding; Genentech: Consultancy; Bristol Meyers Squibb: Consultancy, Research Funding; Janssen: Consultancy. Ding:Merck: Consultancy, Honoraria, Research Funding; AbbVie: Research Funding; DTRM: Research Funding; AstraZeneca: Research Funding; Octapharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Alexion: Consultancy, Honoraria; MEI pharama: Consultancy, Honoraria; BeiGene: Consultancy, Honoraria, Research Funding. Dowling:Gilead: Honoraria; Abbvie: Patents & Royalties; Novartis: Honoraria. Islam:AbbVie: Consultancy; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; BeiGene: Consultancy; DAVA Oncology: Consultancy; MJH Life Sciences: Honoraria; Targeted Oncology: Honoraria; VJHemeOnc: Honoraria. Jain:Novalgen: Research Funding; Loxo Oncology: Research Funding; Dialectic Therapeutics: Research Funding; Kite/Gilead: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Precision Biosciences: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; CareDX: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Mingsight: Research Funding; ADC Therapeutics: Research Funding; Servier: Research Funding; AstraZeneca: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; BMS: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Janssen: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Adaptive Biotechnologies: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; TG Therapeutics: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; MEI Pharma: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES; Ipsen: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES; Pfizer: Research Funding; Medisix: Research Funding; Takeda: Research Funding; Incyte: Research Funding; Aprea Therapeutics: Research Funding; Fate Therapeutics: Research Funding; Beigene: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Pharmacyclics: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; AbbVie: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Genentech: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Newave: Research Funding; TransThera Sciences: Research Funding; Cellectis: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding. Matasar:Seagen: Honoraria, Other: stipends; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Seattle Genetics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Honoraria; Teva: Consultancy; Immunovaccine Technologies: Research Funding; Janssen: Honoraria, Research Funding; Pharmacyclics: Honoraria, Research Funding; Genentech, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Juno: Consultancy; Regeneron: Honoraria, Other: Stipends; Kite: Honoraria, Other: Stipends; Immunovaccine Technologies: Honoraria; Epizyme: Other: Stipends; Celegene: Honoraria, Other: Stipends; BMS: Honoraria, Other: Stipend; Bayer: Consultancy, Honoraria, Research Funding; AstraZeneca: Honoraria, Other: Stipend; Merck: Current equity holder in private company; ADC Therapeutics: Consultancy, Honoraria, Other: Stipend. Miller:AbbVie: Research Funding. Roeker:Pharmacyclics: Consultancy; AbbVie: Consultancy, Research Funding; Janssen: Consultancy; Aptose Biosciences: Research Funding; Ascentage: Consultancy; PeerView: Other: CME speaker; Dren Bio: Research Funding; Adaptive Biotechnologies: Research Funding; Curio: Other: CME speaker; Beigene: Consultancy; Medscape: Other: CME speaker; AstraZeneca: Consultancy, Research Funding; Loxo Oncology: Consultancy, Other: travel support, Research Funding; DAVA: Other: CME speaker; Genentech: Research Funding; TG Therapeutics: Consultancy; Abbott Laboratories: Current equity holder in publicly-traded company; Pfizer: Consultancy, Research Funding; Qilu Puget Sound Biotherapeutics: Research Funding. Rhodes:AstraZeneca: Consultancy; Jannsen: Consultancy; Pharmacyclics: Consultancy, Research Funding; Beigene: Consultancy; GenMab: Consultancy; Oncternal Pharmaceuticals: Research Funding; Acerta: Research Funding; Loxo Oncology: Research Funding; Velosbio: Research Funding; Epizyme: Consultancy, Research Funding; SeaGen: Honoraria; ADC Therapeutics: Consultancy; Morphosys: Consultancy; Genetech: Consultancy; Abbvie: Consultancy, Research Funding. Rogers:Genentech: Consultancy, Research Funding; Loxo@Lilly: Consultancy; Janssen: Consultancy; AstraZeneca: Consultancy; Beigene: Consultancy; Novartis: Research Funding; Pharmacyclics: Consultancy; AbbVie: Consultancy, Research Funding. Shadman:Genmab: Consultancy, Research Funding; Eli Lilly: Consultancy; BeiGene: Consultancy, Research Funding; MorphoSys/Incyte: Consultancy, Research Funding; Regeneron: Consultancy; Vincerx: Research Funding; MEI Pharma: Consultancy; Bristol Myers Squibb: Consultancy, Research Funding; Kite, a Gilead Company: Consultancy; Mustang Bio: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; ADC therapeutics: Consultancy; Fate Therapeutics: Consultancy; Janssen: Consultancy; Genentech: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; TG Therapeutics: Research Funding. Shouse:Beigene, Inc.: Speakers Bureau; Kite Pharmaceuticals: Consultancy, Speakers Bureau. Skarbnik:AstraZeneca: Consultancy, Honoraria, Speakers Bureau; TG Therapeutics: Consultancy, Honoraria, Speakers Bureau; Pharmacyclics: Consultancy, Honoraria, Speakers Bureau; Genmab: Consultancy, Honoraria, Speakers Bureau; Jazz Pharmaceuticals: Honoraria, Speakers Bureau; Beigene: Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria; MorphoSys: Consultancy, Honoraria; Lilly: Consultancy, Honoraria, Speakers Bureau; SeaGen: Consultancy, Honoraria, Speakers Bureau; Kite Pharma: Consultancy, Honoraria, Speakers Bureau; Epizyme: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Speakers Bureau; Genentech, Inc.: Consultancy, Honoraria, Speakers Bureau; Bristol-Myers-Squibb: Consultancy, Honoraria; Alexion: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria, Speakers Bureau; ADC therapeutics: Honoraria, Speakers Bureau. Stephens:AbbVie: Consultancy; AstraZeneca: Consultancy, Research Funding; BeiGene: Consultancy; Bristol-Myers Squibb: Consultancy; Celgene: Consultancy; Genentech: Consultancy; Janssen: Consultancy; Lilly: Consultancy; Novartis: Research Funding. Thompson:Genentech: Research Funding; AstraZeneca: Research Funding; Genmab: Research Funding; MJH Life Sciences: Honoraria; Intellisphere LLC: Honoraria; Brazilian Association of Hematology, Hemotherapy and Cellular Therapy (ABHH): Honoraria; Dava Oncology: Other: Travel ; Curio Science: Honoraria; Massachusetts Medical Society: Honoraria; VJHemOnc: Honoraria; Loxo Oncology at Lilly: Consultancy; AstraZeneca: Consultancy; Janssen: Consultancy; Abbvie: Research Funding; Nurix Therapeutics: Research Funding; Beigene: Research Funding. Thompson:merck: Consultancy, Speakers Bureau; Lilly: Consultancy; janssen: Consultancy, Speakers Bureau; genentech: Consultancy; beigene: Consultancy; astrazeneca: Consultancy, Speakers Bureau; adaptive biotechnologies: Consultancy, Research Funding, Speakers Bureau; abbvie: Consultancy; pharmacyclics: Consultancy. Wang:Novartis: Research Funding; Innocare: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Research Funding; Kite: Honoraria, Membership on an entity's Board of Directors or advisory committees; Genmab: Research Funding; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees; Morphosys: Research Funding; Eli Lilly: Membership on an entity's Board of Directors or advisory committees, Research Funding; LOXO Oncology: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; BeiGene: Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy. Wierda:Nurix THerapeutics: Research Funding; Accutar Biotechnology: Research Funding; NIH P30 CA016672/MDACC Cancer Center Support Grant: Research Funding; Numab THerapeutics: Research Funding; Sunesis: Research Funding; Miragen: Research Funding; Cyclacel: Consultancy, Research Funding; Oncternal Therapeutics, Inc.: Research Funding; KITE Pharma: Research Funding; Janssens Biotech Inc: Research Funding; Loxo Oncology, Inc./Lilly: Research Funding; GlaxoSmithKline: Research Funding; Juno Therapeutics: Research Funding; GSK/Novartis: Research Funding; Janssens Biotech: Research Funding; Bristol Myers Squibb (Juno & Celgene): Consultancy, Research Funding; Gilead Sciences: Research Funding; AstraZeneca/Acerta Pharma: Consultancy, Research Funding; Pharmacyclics LLC: Research Funding; AbbVie: Consultancy, Research Funding; Genentech: Research Funding; National Comprehensive Cancer Network: Other: Nonrelevant Financial Relationship/Chair, CLL). Supported by the NIH/NCI under award number P30 CA016672 and used MDACC Cancer Center Support Grant (CCSG) shared resources. Woyach:Newave: Consultancy; Loxo: Consultancy; Beigene: Consultancy; AstraZeneca: Consultancy; Abbvie: Consultancy; Schrodinger: Research Funding; Morphosys: Research Funding; Karyopharm: Research Funding; Janssen: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal